1. 从下列数据计算平均键能E(C—H):
D(H-C)=340kJ·mo1-1
D(H-CH)=452 kJ·mo1-1
D(C-CH2)=436 kJ·mo1-1
D(H-CH)=436 kJ·mo1-1
答: (340+452 +436 +436 )/4= 416 kJ·mol-1
平均键能E(C—H)= 416 kJ·mol-1
2. 已知
![]()
试计算NH3分子中N—H键的键能。
答: 按题给数据,设计热力学循环
| ΔH4 | ||||
| N2(g) | + | 3H2(g) | ——→ | 2NH3 |
| ↓ΔHl | ↓ΔH2 | ↑ΔH3 | ||
| 2N (g) | + | 6H(g) | ——— |
ΔH1=2ΔfHm˚(N,g)=2×472.70=945.40 kJ·mol-1
ΔH2=6ΔfHm˚(H,g)=6×217.97=1307.82 kJ·mol-1
ΔH4=2ΔfHm˚(NH3,g)=2×(―46.11)472.70=―92.22kJ·mol-1
由ΔH3=―6EN―H
则NH3分子中N—H键的键能为
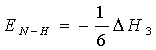
按盖斯定律 ΔH4=ΔHl+ΔH2+ΔH3
则ΔH3=ΔH4―ΔH1―ΔH2=―92.22―945.40―1307.82
=―2345.44kJ·mol-1
![]()